Introduction
Acidity is an important factor affecting the conductivity of aqueous solutions (Covington, Bates and Durst, 1985). However, we need to define the acidity. Thus, the pH scale was developed.
The concept of pH was first introduced by Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909 (Sørensen, 1909) and revised to the current symbol pH at 1929 to keep it consistent with definition and measurements of electrochemical cells. The meaning of pH has been widely disputed. As Nørby (2000) reported, “Current usage in chemistry is that p stands for “decimal co-logarithm of”, as also in the term pKa, used for acid dissociation constants.”
The pH scale is traceable to a set of standard solutions whose pH is established by international agreement (Covington, Bales and Durst, 1985). Precise measurement of pH is presented in International Standard Organization ISO 31-8 as follows: A galvanic cell is setup to measure the electromotive force (e.m.f.) between a reference electrode and an electrode sensitive to the hydrogen ion activity when they are both immersed in the same aqueous solution. The reference electrode may be a silver chloride electrode or a calomel electrode. The hydrogen-ion selective electrode is a standard hydrogen electrode (ISO, 1992)
The pH is defined as the decimal logarithm of the reciprocal of the hydrogen ion activity, aH+, in a solution. 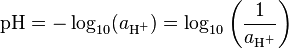
This definition is adopted because ion-selective probes, which are used to measure pH, respond to activity. Ideally, electrode potential, E, follows the Nernst equation, which, for the hydrogen atom can be expressed as follows: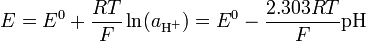
where E is a measured potential, E0 is the standard electrode potential, R is the gas constant, T is the temperature in kelvin, F is the Faraday constant.
Now, lets go on to how a pH probe is calibrated.
Firstly, the cell is filled with a solution of known hydrogen ion activity and the emf, ES, is measured. Then the emf, EX, of the same cell containing the solution of unknown pH is measured.
The difference between the two measured emf values is proportional to pH. This method of calibration avoids the need to know the standard electrode potential. The proportionality constant, 1/z is ideally equal to  the "Nernstian slope" (Covington, Bates and Durst, 1985)
the "Nernstian slope" (Covington, Bates and Durst, 1985)
However, to apply this process in practice, a glass electrode (which is what is used in commercially available sensors nowadays) is used rather than the cumbersome hydrogen electrode. A combined glass electrode has an inbuilt reference electrode (to satisfy the equation previously). It is calibrated against buffer solutions of known hydrogen ion activity. IUPAC has proposed the use of a set of buffer solutions of known H+ activity (Covington, Bates and Durst, 1985) Two or more buffer solutions are used in order to accommodate the fact that the "slope" may differ slightly from ideal (therefore the need to have a pH 4 and pH 10 solution). To implement this approach to calibration, the electrode is first immersed in a standard solution of pH 7 and the reading on a pH meter is adjusted to be equal to the standard buffer's value. The reading from a second standard buffer solution is then adjusted, using the "slope" control, to be equal to the pH for that solution. Further details, are given in the IUPAC recommendations (Covington, Bates and Durst, 1985) When more than two buffer solutions are used the electrode is calibrated by fitting the observed pH values to a straight line with respect to standard buffer values. Commercial standard buffer solutions usually come with information on the value at 25 °C and a correction factor had to be applied for other temperatures due to the fact that the pH would be affected.
From the above you can see that acidity plays a vital role in many science projects. It can be both a useful and dangerous substance depending on how you use it. The pH sensor aids to measure the acidity of an aquarium or other liquids. Also, it is of importance in aquaponics, where crucial management of the acidity, amongst other factors, its of utmost importance. It has played an important role in many scientific discoveries too.
1.1 Engineering Problem
Maintaining a constant pH is important in many situations. For example, it is necessary to control the pH of an aquarium as certain species of fish can only live within a set pH range. Other fields where controlling the pH is important include hydroponics, fermentation processes like beer and wine production, environmental monitoring of soils, sewage treatment tanks, monitoring of solution and buffers in chemistry laboratories. It is quite difficult to find good and cheap pH sensors. So we have decided to build a cheap and simple pH sensor with easily accessible materials. We will be build it so it can fit in a box with a small display making it portable. Building a small and cheap pH sensor will benefit many people by making pH sensor a widely available product. Also, we aim to make the pH sensor easy to fix, reducing the hassle of having to find a replacement after the pH meter has been damaged.
1.2 Engineering Goal
a) Minimise the size of the pH meter in order to increase the reliability and affordability.
b) Expand the scope of pH and more research to be conducted.
c) To develop a cheap pH sensor from buying the Sparkfun Inventor's kit and combining it with the Arduino to come out with a integrated system.
1.3 Specific Requirements
- Must fit in a box the size of a typical scientific calculator
- Must be portable and easy to carry about
- Must be able to run on power from both wall plug and 2 x 9V rectangular battery (which is a replaceable power source)
- Must be reliable (Accuracy must match that of commercial sensor)
- Build a reliable digital pH meter for a fraction of the cost of an expensive industrial ph meter or benchtop pH meter
1.4 Alternative solutions
Litmus paper
How it works:
Litmus is a water-soluble mixture of different dyes extracted from lichens, primarily Roccella tinctoria. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity (Neupert, 2013). Litmus paper, then, it paper with litmus powder, which reacts to different acidity levels. Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic (i.e. alkaline) conditions while neutral litmus paper is purple (Neupert, 2013) and turns into red or blue depending whether it is basic or acidic conditions.
Litmus was used for the first time about 1300 AD by Spanish alchemist Arnaldus de Villa Nova (Neupert, 2013).
Advantages
- Cheap and affordable
- Widely available
Disadvantages
- Inaccurate, therefore not suitable for situations that require precise values.
- e.g.: aquatic industry, where certain species of fish are adapted to live in certain pH values
Conductivity of the medium
How it works:
The conductivity of the medium is measured, then pH is calculated from that using the mathematical functions and correlations between the values. The conductivity of the medium is measure using a conductivity sensor.
The conductivity sensor, also known as a electrical conductivity meter, is a common laboratory conductivity meters that employs a potentiometric method and four electrodes. Often, the electrodes are cylindrical and arranged concentrically and are usually made of platinum metal. Following that, an alternating current is applied to the outer pair of the electrodes and the potential between the inner pair is measured. Conductivity could in principle be determined using the distance between the electrodes and their surface area using the Ohm's law. However, for accuracy, a calibration is employed using electrolytes of well-known conductivity.
Industrial conductivity probes often employ an inductive method, which has a distinct advantage that the fluid does not wet the electrical parts of the sensor. Here, two inductively-coupled coils are used instead of using a potentiometric method and four electrodes. One is the driving coil producing a magnetic field and it is supplied with accurately-known voltage. The other forms a secondary coil of a transformer. The liquid passing through a channel in the sensor forms one turn in the secondary winding of the transformer. The induced current is the output of the sensor, which is the conductivity.
Advantages:
- For people that need to know pH without having to go through the hassle of calibrating the sensor, they can use this method for its versatility. It is called the inferred pH, calculated from the cation conductivity and normal conductivity.
Disadvantages:
- Overly complicated when pH can be taken with a normal conventional sensor
- Require advance technical know-how to perform this method. Does to suit a layman looking for a simple way to take pH values
- Requires the use of other sensors which defeat the purpose of this method (it beats around the bush when you can just use a pH sensor)
Usage of living organisms as indicators of pH value:
How it works:
By using living organisms who are naturally sensitive to the pH values as indicators, we can effectively gauge when the pH values is surpassing certain values as this would most probably cause adverse effects in the organisms, including death, that could be easily seen and observed with the naked eye.
Advantages:
- Easy to see and gauge.
- Easy to implement.
- Effective at gauging pH values
- Simple to implement
- Does not require any prior preparation if required wildlife is found in the environment beforehand
Disadvantages:
- Unethical, subjecting animals to unnecessary harm.
- Is not very accurate at providing specific values, only vague ones.
- Controversial (in the same way as animal testing)
- Requires special license from certain governing bodies in certain countries (in the form of paperweight)
- Need to take special care of the animals to ensure their survival in the environment therefore adding to additional cost
Usage of pH probe:
How it works:
A typical modern pH probe is a combination electrode, which combines both the glass and reference electrodes into one body.
The combination electrode consists of the following parts (see the Figure 1):
- The sensing part of electrode, a bulb made from a specific glass
- Internal electrode, usually silver chloride electrode or calomel electrode
- Internal solution, usually a pH 7 buffered solution of 0.1 mol/L Potassium Chloride(KCl) for pH electrodes or 0.1 mol/L MeCl for pMe electrodes
- When using the silver chloride(AgCl) electrode, a small amount of AgCl can precipitate inside the glass electrode
- reference electrode, usually the same type as 2
- reference internal solution, usually 0.1 mol/L KCl
- junction with studied solution, usually made from ceramics or capillary with asbestos or quartz fiber.
- body of electrode, made from non-conductive glass or plastics.
The bottom of a pH electrode balloons out into a round thin glass bulb. The pH electrode is best thought of as a tube within a tube. The innermost tube (the inner tube) contains an unchanging 1×10−7 mol/L Hydrogen Chloride(HCl) solution. Also inside the inner tube is the cathode terminus of the reference probe. The anodic terminus wraps itself around the outside of the inner tube and ends with the same sort of reference probe as was on the inside of the inner tube. It is filled with a reference solution of 0.1 mol/L KCl and has contact with the solution on the outside of the pH probe by way of a porous plug that would serve as a salt bridge
Advantages:
- Simple and functional
- Does not require much set-up
- Provides fast and accurate reading
- Widely available at low-cost
- Can be used in a wide variety of settings
Disadvantages:
- Requires calibration beforehand
- Needs special care in storage
- Fragile
- Needs to be calibrated infrequently (once in a year)
1.4.4 Final Solution
After taking into consideration the simplicity, price and accuracy, we choose to use a commercially available pH probe and create a pH meter to fully utilize its abilities. The commercially available pH meter excels in all the areas consider. It is simple to use (it only requires calibration to ensure its reliability and usability), it is cheap to use (a pH probe can be had for as low as $9.99 on Amazon) and, most of all, its accuracy is the best out of the all the alternatives considered. Thus, we decided to use a pH probe and make a pH meter.
No comments:
Post a Comment